 gasoline
gasoline
 powers cars and trucks
powers cars and trucks kerosene
kerosene
 jet fuel
jet fuel natural gas
natural gas
 heats homes
heats homes coal and fuel
coal and fuel
 generate electricity
generate electricity
Lecture VI
Chemical Energy is the
root source of energy used for
transportation and
industry
The electric energy we use comes in large part from chemical processes. Many of the chemical compounds used to produce energy are burned and involve chemical changes:
 gasoline
gasoline
 powers cars and trucks
powers cars and trucks kerosene
kerosene
 jet fuel
jet fuel natural gas
natural gas
 heats homes
heats homes coal and fuel
coal and fuel
 generate electricity
generate electricity
A basic understanding of chemical change is thus central to any discussion of
 energy
energy energy production.
energy production.
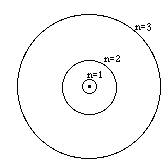
Atoms The atom is the building block of all molecules and complex
chemicals.
 protons
protons
 neutrons
neutrons
 electrons.
electrons.
 10-10 m in diameter.
10-10 m in diameter.
 protons
protons neutrons.
neutrons.
The chemical behavior of atoms is determined by the number and the configuration of the electrons in the atom.
Why should one bother to discuss the nucleus?
The number of protons in the nucleus determines the number of electrons in the atom.
More on Atoms Most atoms in the world around us are electrically neutral.
They have no net charge.
 protons are positively charged
protons are positively charged
 electrons are negatively charged
electrons are negatively charged
 charge of a proton = -charge of
electron
charge of a proton = -charge of
electron
The presence of 10 protons in a nucleus
 the presence of 10 electrons to
balance the charge.
the presence of 10 electrons to
balance the charge.
The same is true of any nucleus.
The simplest atom, hydrogen, has just one proton in the nucleus.
The next atom has two protons in the nucleus...
Atoms are designated by symbols [see periodic table].
Hydrogen's symbol is H. Other important atomic symbols are carbon, C, nitrogen, N, and oxygen, O.
A bit of the Periodic Table (!!!)
| H | He | ||||||
| Li | Be | B | C | N | O | F | Ne |
Atoms can also be designated by the number of protons in them (the
proton number or atomic number is given the symbol Z).
The number of neutrons--or, actually, protons plus neutrons--is also
important. The mass number, A, is the total number of protons
and neutrons in an atom's nucleus. The type of atom is then determined by giving its Z and A.
Chemical notation Example: Z = 1 already tells us that it's hydrogen; The properties of atoms are determined by Quantum Mechanics.![]() is redundant.
is redundant.
 ordinary hydrogen is designated
ordinary hydrogen is designated
![]()
 ordinary carbon is designated
ordinary carbon is designated
![]() .
.
Z = 6, that it's carbon.
Quantum Theory of the Atom Why do we see atomic spectra?
Why are there stationary states?
Why is there quantum mechanics? electrons have wave
properties
electrons have wave
properties
![]()
 for electron to be in a stable
orbit
for electron to be in a stable
orbit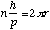 or
or
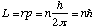
 what is the energy of the
stationary state
what is the energy of the
stationary state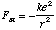
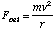
Radius: 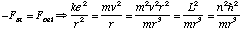
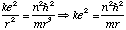
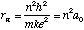
Energy: 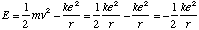
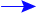
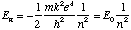
Types of Chemical Reactions The formation of a compound occurs when the compound is more stable
than the constituents were when they were alone. There are two different
sorts of chemical reactions those that take
in energy - endothermic
those that take
in energy - endothermic
 those that give
off energy - exothermic
those that give
off energy - exothermic
Exothermic reactions result when the chemical bonds between atoms in molecules are reformed or when the overall result of a chemical reaction is the making of bonds.
The reactants fall into their most stable available state by giving up energy. These bonds all involve atomic electrons. The nuclei of the constituents remain unchanged.
Problem of the day What is the residence time of H2O in
the Earth's atmosphere? Once again we will use a steady-state model....so what do we expect? And what do we need?
 The amount of water M in the
atmosphere is:
1.3x1013m3
The amount of water M in the
atmosphere is:
1.3x1013m3
 The global precipitation rate
Fw is:
5.2x1014m3/yr
The global precipitation rate
Fw is:
5.2x1014m3/yr
Then the residence time is:
T = M/Fw
T = 1.3x1013m3/5.2x1014m3/yr
T= 0.025 yr
T = 9 days
Does this agree with what we expect?