Lecture VII
Physics 367
Chemical Energy and Energy Generation
Types of Chemical Reactions
The formation of a compound occurs when the compound is more stable
than the constituents were when they were alone. There are two different
sorts of chemical reactions
 those that take
in energy - endothermic
those that take
in energy - endothermic
 those that give
off energy - exothermic
those that give
off energy - exothermic
Exothermic reactions result when the chemical bonds between
atoms in molecules are reformed or when the overall result of a chemical
reaction is the making of bonds.
The reactants fall into their most stable available state by
giving up energy. These bonds all involve atomic electrons. The
nuclei of the constituents remain unchanged.
To understand this think of a golf ball falling into a hole in the
ground. It is more stable than that same golf ball on the ground, because
the one on the ground could fall into the hole. The one in the hole can
fall no farther. It gave up gravitational energy to fall into the hole.
To bring it back out off the hole involves payment of an energy price.
The ball must gain as much energy as it lost going into the hole to get
out again.
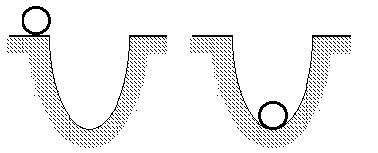
A golf ball in a hole.
In a similar way, chemicals before reacting have a certain amount of
energy. They may react together and give up energy by making bonds among
the atoms (or constituents). Thus, they "fall into a hole," so to speak.
To restore them to their original states, energy must be supplied--the
same amount they gave up to form the compound.
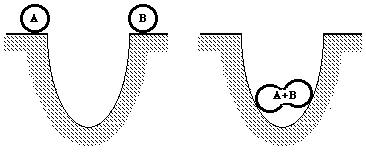
Formation of a chemical compound
The falling into the hole part involves the giving off of energy
during the making of chemical bonds. It is exothermic. The
getting out of the hole part involves the absorption of energy during
the breaking of chemical bonds. It is endothermic.
Activitation Energy
To get a reaction to start, even one that produces useful energy, it
is necessary to give some energy to the system.
The energy provided to start a reaction going is called activation
energy.
Example: Water is formed from hydrogen
gas and oxygen gas.
If we put them into a container and waited, there would be a very slow
reaction. After thousands of years, molecules would break into atoms,
atoms would hit appropriate other atoms as they jostle about with their
thermal energy, and the compound would form.
However, supplying a small amount of activation energy [with a match,
say] starts off a reaction. A large number of molecules break into
atoms near the matchhead. The atoms combine to form water, which
liberates a large amount of energy. This energy supplied breaks up
molecules farther away, they combine, until the whole volume of gas
is water. This sort of reaction is called a chain reaction.
When chemicals burn, that is, combine with oxygen in the air, they
form bonds and release energy. Combustion involves liberation of
energy (usually as thermal energy) - it is an
exothermic process. The heat of
combustion is the amount of energy given off per kilogram of the
substance burned. Various heats of combustion are listed below:
| Substance |
H of C (MJ/kg) |
H of C (kWh/kg) |
| Fuel - crude oil |
45.0 |
12.5 |
| - gasoline
|
46.9 |
13.0 |
| - kerosene
|
46.7 |
13.0 |
| - Ohio coal
|
29.5 |
8.2 |
| - hydrogen
|
141.9 |
39.4 |
| Foods - butter |
38.5 |
10.7 |
| - animal fats
|
37.8 |
11.0 |
| - egg white
|
23.9 |
6.6 |
| - egg yolk
|
33.9 |
9.4 |
| - olive oil
|
39.3 |
10.9 |
| Other - oak |
16.7 |
4.6 |
| - pine
|
18.5 |
5.1 |
| - dynamite
|
5.4 |
1.5 |
| - iron
|
6.6 |
1.8 |
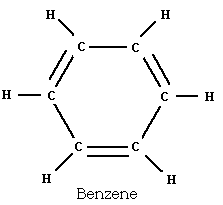
Example: the transformation of the organic
chemical benzene, a liquid, into the solid explosive TNT.
Benzene is obtained from coal tar (it is also found in crude oil in
very small amounts). Benzene is shown below:
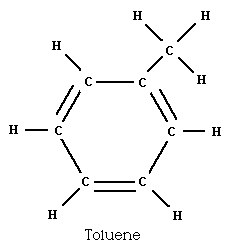
The replacement of one branch from a carbon-to-a-hydrogen bond with
a carbon-to-a-(carbon with three hydrogen) bond forms the liquid
toluene.
If another carbon-to-hydrogen bond in toluene is replaced by a
carbon-to-(nitrogen with two oxygens), nitrotoluene is formed.
If this replacement is accomplished for three carbon-to-hydrogen bonds,
trinitrotoluene (TNT) is formed
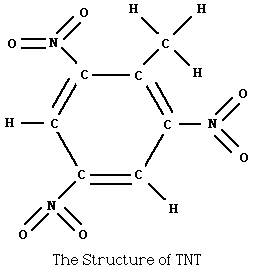
This replacement takes energy [toluene is added at 120°C to
nitric acid, sulfuric acid, and SO3 to form TNT]. The
resulting chemical is a pale yellow solid at room temperature.
TNT melts at 83°C, and explodes at 240°C. Supplying TNT
with activation energy produces N2, CO, CO2,
H2O, and lots of energy in a chemical chain reaction.
Catalysis
It is possible to change the rate at which reactions occur. This
process is known as catalysis.
Catalysts are the materials which speed up the reaction but
which are not used up or even affected in the reaction. In living things
catalysts called enzymes make life possible.
Depletion of the ozone layer is made possible by catalysts containing
chlorine and bromine:
ClO + BrO 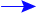 Cl + Br + O2
Cl + Br + O2
Cl + O3 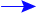 ClO + O2
ClO + O2
Br + O3 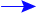 BrO + O2
BrO + O2
Energy Generation
Carbon compounds play a vital role in the existence of life on
Earth. Plants inhale carbon dioxide, exhale oxygen and store energy in
glucose....animals reverse this process.
6CO2 + 6H20 + radiant energy
 C6H12O6 + 6O2
C6H12O6 + 6O2
Carbon atoms also form most fuels known:
 natural gas
natural gas
 coal
coal
 oil.
oil.
The branch of chemistry studying carbon is known as organic
chemistry because carbon is also the atom of life. Carbon can form
more complicated compounds than most other atoms.
Chemical change by burning is involved in most of the methods we use to
generate electricity. Fossil fuel contains carbon, and it is burned by
combining with oxygen in the air. Air is about 20% oxygen and 80% nitrogen.
The combustion converts chemical energy stored in the fuel to kinetic energy
[thermal energy]. Since fossil fuels contain hydrogen in addition to carbon,
combustion also produces water.
But the process is not that simple
!
What are the problems associated with burning fossil fuels?
Problems
 Sulfur
Sulfur
 Nitrogen
Nitrogen
 Heat
Heat
 Particulates
Particulates
Most power plants are cooled by water, which is heated and then
returned hotter to the source of the water. This may cause
deleterious effects on fish which can be injured or killed if the
temperature is too high. They may also fail to reproduce, even at
relatively low temperatures.
This exhaust of this waste heat
is another pollutant emitted by power plants.
The chemicals that burn are made of other things. We call these
molecules, or collections of atoms that are bound together. Every
time we burn a chemical we produce byproducts in addition to heat. Every
breath you take you breathe in several liters of air. There are about
1023 air molecules in that amount of air. Even a small
contamination of certain particulates can destroy your lungs.
This exhaust of this particulates
is another pollutant emitted by power plants.
Absolute temperatures
All particles--atoms, molecules, and so on--jitter about at room
temperature because they have thermal energy. Now we have to think about
what temperature means.
We are used to the Celsius temperature scale. That scale is
defined by:
0°C = the temperature at which ice freezes
100°C = the temperature at which water boils [at sea level]
Using this scale a day with below zero temperatures is cold. When you
drink a soft drink full of ice, the temperature is about 0°C. A day
with 10°C temperatures would be brisk--you would wear a jacket. This
is also the temperature of a cold beer. Room temperature is about
20°C. Your skin temperature is about 35°C and your internal
body temperature is 37°C. A day with temperatures in the 30s would
be hot. You eat hot foods at temperatures in the 60s.
That scale is fine for human affairs, but fails to be truly universal
because it's based on Earthly conditions [sea level pressure].
Also, clearly, objects at temperatures below zero have thermal energy.
We would like a scale that would register zero when objects have
zero thermal energy. The absolute temperature
scale is one that has its zero at this temperature. The absolute
zero of temperature is 273° below zero Celsius. The size of the unit
of temperature is set to be the same as 1°C. The unit is called the
kelvin(K) in honor of Lord Kelvin.
Thus, Table 1 shows comparisons.
| Celsius Temperature |
Absolute Temperature |
| -273°C |
0 K |
| 0°C |
273 K |
| 10°C |
283 K |
| 20°C |
293 K |
| 37°C |
310 K |
| 100°C |
373 K |
Temperature is a measure of an objects thermal energy [kinetic energy].
The distribution referred as the speeds of gas particles leads to the
connection of the thermal energy to temperature.
Problem of the day:
A stable and highly soluble pollutant is dumped into a lake at the rate
of 0.16 tonnes per day (0.16x103 kg per day). The lake volume
is 4x107 m3 and the average water flow through the
lake is 8x104 m3/day. Ignore evaporation (we will
discuss this later) from the lake surface and assume the pollutant is
uniformly mixed in the lake. What eventual
steady-state concentration will the pollutant reach?
Once again we will use a steady-state model....so what do we expect?
And what do we need?
 The amount of water in the lake,
Mw is:
4x107 m3
The amount of water in the lake,
Mw is:
4x107 m3
 The average water flow through the
lake, Fw is:
8x104 m3/day
The average water flow through the
lake, Fw is:
8x104 m3/day
Then the residence time of the water in the lake is:
Tw = Mw/Fw
Tw = 4x107 m3/
(8x104 m3/day)
= 0.5x103 day
Tw = 500 days
How do we calculate the residence time of the pollutant?
Because the pollutant is uniformly mixed
in the lake, the residence time of the pollutant will equal the residence
time for the lake water.
Tw = Tp = 500 days
Then it follows that the steady state stock of pollutant is simply the
input rate times the residence time:
Mp = FpTp
= 0.16 tonnes/day x 500 days
Mp = 80 tonnes
To get the concentration we need to know how much water there is in
the lake in tonnes. If we multiply the volume of water by the density
of water (1g/cm3 = 1kg/1000cm3 = 1000 kg/m3
we find that 1 cubic meter weighs exactly 1 metric ton (1000 kg) which
is equal to 1 tonne. The the steady state concentration of the pollutant
is:
Cp = 80 tonnes/4x107 tonnes
= 2x10-6
Cp = 2 parts per million
by weight
 those that take
in energy - endothermic
those that take
in energy - endothermic those that give
off energy - exothermic
those that give
off energy - exothermic




 Cl + Br + O2
Cl + Br + O2 C6H12O6 + 6O2
C6H12O6 + 6O2 natural gas
natural gas Sulfur
Sulfur