Components for Organic-Based Magnets
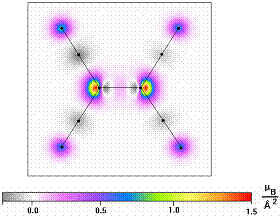
Organic-based magnets are prepared using solution- or vapor-phase methods associated with organic chemistry and not metallurgical or ceramic engineering technologies. The organic molecule units of organic-based magnets play a crucial role in magnetic ordering by providing essential unpaired electron spins and/or a means of mediating the interaction between the units with unpaired spin. The spins provided by organic molecular units are associated with electrons in p, π, or s orbitals, in contrast to the d and f orbitals involved in transition-metal and rare-earth-based magnets, respectively. The organic molecular units have extended spatial shapes that can lead to local structural order and longrange crystal structures that are highly anisotropic, with differing magnetic interactions in different directions. The organic molecules in organic-based magnets have an internal electronic structure with a mix of fully occupied, partially occupied, and empty molecular orbitals. These features result in new phenomena that are unknown or rare for conventional magnets. These phenomena include light-induced magnetism at relatively high temperatures;1 "fully spin-polarized" magnetic semiconductors, or "halfsemiconductors,"2 that is, semiconductors for which the valence band (or HOMO level) is occupied only with electrons that have all spins pointing in one direction and for which any electrons that enter the otherwise empty conduction band (or LUMO level) have opposite spin direction to the electrons in the valence band); and fractal magnetic order.3 This article discusses these phenomena in later sections. Key building blocks for organic-based magnets include the organic electron acceptor tetracyanoethylene (TCNE) (see figure). The spin of the unpaired electron on the charged anion radical [TCNE]•- resides primarily on the two central carbon atoms and the external four nitrogens in a π* orbital that spreads over the molecule.4

[TCNE]- spin density distribution measured by polarized neutron diffraction. A. Zheludev, A. Grand, E. Ressouche, J. Schweizer, B.G. Morin, A.J. Epstein, D.A. Dixon, and J.S. Miller, Journal of the American Chemical Society 116, 7243 (1994).
1. D.A. Pejakovic, J.L. Manson, J.S. Miller, and A.J. Epstein, Physical Review Letters 58, 057202 (2002).
2. V.N. Prigodin, N.P. Raju, K.I. Pokhodnya, J.S. Miller, and A.J. Epstein, Advanced Materials 14, 1230 (2002).
3. S.J. Etzkorn, W. Hibbs, J.S. Miller, and A.J. Epstein, Physical Review Letters 89, 207201 (2002).
4. A. Zheludev, A. Grand, E. Ressouche, J. Schweizer, B.G. Morin, A.J. Epstein, D.A. Dixon, and J.S. Miller,
Journal of the American Chemical Society 116, 7243 (1994).
Return to the main page
Webpage created 25 November 2003 by John Rohrbacher
Contact the webmaster