In systems where all of the degrees of freedom can be defined by a single temperature, rotational spectroscopic studies of linear and polyatomic molecules ordinarily have been done in the regime hνr << kT, for thermally populated levels Jmax ~10 - 100 and partition function Qr ~ 102 - 105. In this regime, there is in general spectral complexity, and for collisions a multitude of "open" channels, with "classical" collision properties which result from averaging over these many channels.
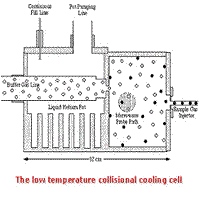
The temperature effect on collisions can be dramatic. At 1 K the orbital angular momentum of a He atom colliding with CO is L ~ 2 and the collisional channels associated with the J = 1 rotational state of CO are energetically marginally available. However, at 300 K, the orbital angular momentum associated with the collision is L ~ 30 and a multitude of collisional channels through J = 10 are open. The effect of the increase in open channels can be seen in Fig IV-1, which shows the calculated pressure broadening cross section for the J = 1 - 0 transition of CO in He at 115 GHz as a function of collision energy. At low temperature (2 cm-1 ~ 3 K) individual resonances, associated with the formation of quasi-bound states, are observable in the pressure broadening cross section (as well as in the state-to-state rates and the pressure shifts), while at higher temperature, the resonances slowly merge and disappear and the cross section over a very wide temperature range is reduced to essentially that of the classical "size" of the molecules. Because of our desire to explore these and related phenomena, we have sought to develop a general, expandable methodology to study the regime for which hνr ≤kT, Jmax ~1 - 5, and Qr ~ 1 - 10. In this regime, there is not only significant spectral simplification, but also a much closer and more interesting relation between experimental observables and fundamental molecular parameters. Additionally, in the millimeter and submillimeter (mm/submm) spectral region hν r ~ hνexp ~ kT, and this spectral region is especially advantageous for many scientific studies. In the low temperature, non-equilibrium interstellar medium, rotationally inelastic collisions between assorted heavy molecules and the dominant gases, H2 and He, are critical in determining the rotational state distributions of the molecules and the interpretation of the astronomical data themselves. These topics are considered in more detail in (442)
 The Collisional Cooling (CC) of Neutrals--an experimental technique
The Collisional Cooling (CC) of Neutrals--an experimental technique
Over the past 25 years we have developed and characterized a simple methodology for the study of gas phase molecules at temperatures far below their freezing points.(386, 423, 425, 435 , 439, 449, 456) This is accomplished by injecting small amounts of warm, spectroscopically active gas into cells filled with cold He or H2. Because at typical spectroscopic pressures the warm gasses collisionally cool to the temperature of the background gas in far fewer collisions than required to reach a wall (where they condense), these collisionally cooled (CC) molecules are attactive subjects for a wide range of studies.
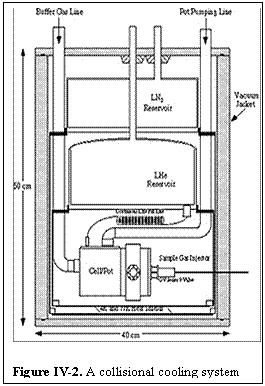
As an example, Fig.IV-2 shows the layout of one of the collisional cooling systems. The entire apparatus is in a vacuum chamber maintained at a pressure below 10 -5 Torr. Shields at 77 K (maintained by liquid nitrogen) and at 4 K (maintained by liquid helium) surround the region which contains the collisionally cooled cell. This cell is connected to a helium pot which is filled via a continuous fill capillary from the helium reservoir and pumped via an external pumping line. The cell is made of oxygen-free high conductivity copper and has 5 mil mylar windows. All removable flanges are sealed with indium. The temperature of the cell is varied by pumping on the pot through a vacuum regulator valve, which accurately controls the pressure and thus the temperature of the liquid helium. Since in this system the cell is isolated from the helium reservoir, temperatures above 4.3 K are also readily maintained. This technique allows the straightforward variation and control of the temperature, which is read directly with calibrated germanium resistance thermometers. The cell pressure is measured by a capacitance manometer at room temperature, with a correction for the effects of thermal transpiration.
At the side of the experimental chamber, warm spectroscopically active gas is injected via a vacuum insulated tube. Provisions exist for electrically heating this tube, but under normal conditions this has not been necessary for the relatively volatile gases used. The collisionally cooled cell is first filled with a static pressure of helium or hydrogen in equilibrium with the wall temperature. The spectroscopically active gas then cools as it collides with the cold buffer gas. The quantitative relations that make this a useful and general technique are: (1) the injected gas cools very rapidly as it collides with the buffer gas, requiring far fewer than 100 collisions to closely approach the temperature of the background; (2) at typical pressures, about 10 000 collisions are required for the gas to reach the walls and to condense; and (3) the very large absorption coefficients characteristic of very low temperature spectroscopy make possible large dilution ratios that ensure the concentration of the spectroscopic gas is so small as to not perturb the temperature of the buffer gas. In order to avoid the corrections to lineshape that are associated with large absorption, the flow rate is typically adjusted to produce an absorption of 1% - 10%. This corresponds to dilution ratios at temperatures around 4 K (depending upon the absorption coefficient of the gas) of ~ 10-4 - 10-6.
Collisional cooling has now been used by a number of laboratories in a rather wide variety of applications. In an experiment which combined a collisionally cooled system built by Dan Willey's group at Allegheny College with the infrared lasers of George Flynn's group at Columbia, Willey et al. have extended the technique into the infrared and shown that both the translational temperature and rotational temperature are consistent with the measured wall temperature at the somewhat higher pressures and injector flow rates appropriate for infrared studies. (Willey, et al., 1995a) At Brookhaven, Trevor Sears' group has developed a multipass system for the infrared and in their initial experiments have studied N2O, NO, and NO2 near 5 K. (Jin, et al.) They were able to establish that both the rotational and translational temperatures were that of the cell walls, and were able to take advantage of the significantly reduced Doppler widths to resolve hyperfine structure. They also expect to be able to study the evolution of chemically reactive species following laser initiated photochemistry. Miller's group at North Carolina has adapted the technique for the study of aerosols and found that they can vary the size distribution over a wide range by simple adjustment of cell pressure, gas composition and flow rate (Dunder, et al.) In another collaborative experiment a collisionally cooled system built by Willey's group has been used in conjunctions with the a special high spectral purity diode laser system developed by Arlan Mantz at Franklin and Marshal to study CH3F at 7.5 K. In contrast to the other experiments, this work determined rotational and translational temperatures about twice that of the cell temperature. This result underscores the fact that these are quasiequilibrium experiments and that if collisionally cooled systems are to be used in collisional studies which require a known and controlled kinetic collision temperature, then it is important to understand the thermodynamics of the system and to do careful diagnostics. In the United Kingdom, this method has also been used in conjunction with an FTIR by Ballard and Newnham, who are interested in upper atmospheric problems. Likewise, in related work we have developed liquid nitrogen cooled systems for mm/submm studies related to the atmosphere of the earth and other planets.(439, 444) Finally, in an especially interesting experiment Willey's group has shown maser action in a collisionally cooled cell without any additional pump energy. (Willey, et al., 1995b) In this experiment differential rotational relaxation of the initially warm NH3 in collision with cold He produces a significant population inversion on the (J,K = 4,3) transition.
| OSU Physics Department | The College of Math and Physical Sciences | The Ohio State University |