Collisional Studies of Neutral Species at Very Low Temperature
In traditional collisional studies, the large number of thermally
populated states and the nature of the molecular interaction cause much of the
complexity. Thus, it is expected that the very large reduction in the number
of thermally populated states at low temperature will make the relation
between observables and the fundamental interactions significantly more
direct.
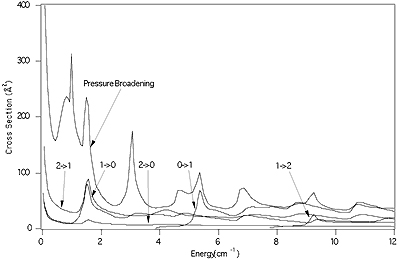 A substantial body of experimental pressure broadening data has now been
obtained by use of the collisional cooling technique
(
386, 411, 413, 418, 419, 420, 423, 441, 442, 449, 456) for CO,
H2S, HDO, CH3F, and NO in
collision with He and H2. For a few of these intermolecular
potentials obtained either by ab initio techniques or from the analyses of the
spectra of weakly bound complexes are available and theoretical methods (e.g.
MOLSCAT) make possible a direct
comparison between experiment and theory.
A substantial body of experimental pressure broadening data has now been
obtained by use of the collisional cooling technique
(
386, 411, 413, 418, 419, 420, 423, 441, 442, 449, 456) for CO,
H2S, HDO, CH3F, and NO in
collision with He and H2. For a few of these intermolecular
potentials obtained either by ab initio techniques or from the analyses of the
spectra of weakly bound complexes are available and theoretical methods (e.g.
MOLSCAT) make possible a direct
comparison between experiment and theory.
Due to the fundamental role played by CO - He in quantum chemistry as
well as astrophysics, this system has been the subject of extensive study
(Green and Thaddeus)
(Green and Palma)
(Cohen et al.)
(Korona, et al.).
The figure shows a comparision between
experimental results and MOLSCAT
calculations based on an early ab initio intermolecular potential (IMP) due to
Thomas, Kraemer, and Dierksen
(Dierksen, Kraemer, and Thomas)
and a more recent IMP derived from the spectrum of the CO - He complex
(Chuaqui, Le Roy, and McKellar).
Return to
The Microwave Laboratory
<OSU Physics Department
|The College of Math and Physical
Sciences
|The Ohio State University
>
 A substantial body of experimental pressure broadening data has now been
obtained by use of the collisional cooling technique
(
386, 411, 413, 418, 419, 420, 423, 441, 442, 449, 456) for CO,
H2S, HDO, CH3F, and NO in
collision with He and H2. For a few of these intermolecular
potentials obtained either by ab initio techniques or from the analyses of the
spectra of weakly bound complexes are available and theoretical methods (e.g.
MOLSCAT) make possible a direct
comparison between experiment and theory.
A substantial body of experimental pressure broadening data has now been
obtained by use of the collisional cooling technique
(
386, 411, 413, 418, 419, 420, 423, 441, 442, 449, 456) for CO,
H2S, HDO, CH3F, and NO in
collision with He and H2. For a few of these intermolecular
potentials obtained either by ab initio techniques or from the analyses of the
spectra of weakly bound complexes are available and theoretical methods (e.g.
MOLSCAT) make possible a direct
comparison between experiment and theory.