I. INTRODUCTION
The genesis of microwave, and ultimately THz spectroscopy, was the war time development of microwave radar. However, this development was greatly aided by a fortuitous (for microwave spectroscopy at least!) accident that placed a previously unknown transition of water (the 616-523 at 22 GHz) in the middle of the spectral region that was being developed at the end of the war as the next new radar band. Not only did this make vast quantities of sophisticated equipment that ordinarily would have been beyond the means of university researchers immediately available, but it also established the 'relevance' of the field.
By 1948 the field was mature enough that an article in Reviews of Modern Physics [1]. appeared and by 1952 another review entitled Microwave Spectroscopy above 60kMc reported work to above 125 GHz [2] .Technology advanced rapidly and by 1954 the submillimeter threshold at 300 GHz had been passed [3]. This drive toward ever higher frequencies was aided by the rapidly increasing absorption strengths of the spectra of many of the most important small fundamental molecules (e. g. H2O, O2, CO, HCl, and O3) which lay there.
A strong argument can be made that this scientific focus led spectroscopists towards the development of practical and robust ‘THz’ technologies, including the crossed waveguide harmonic generator [4], electronic frequency control systems [5], quasi-optical propagation, and the exploitation of sensitive detectors to compliment the harmonic generation sources [6].
While microwave spectroscopy had a number of other streams, in the end this drive to higher frequency to observe the most fundamental species provided the enduring legacy for the field. Because these small species were not only scientifically fundamental but also pervasive in many physical and chemical systems, the strong interactions in the THz region have led to a number of important applications. In later sections we will discuss some of them. At appropriate places links are included to particularly useful web sites which can be consulted for additional information.
A. Radiation and Matter: At a fundamental level what distinguishes one spectral region from another is how radiation interacts with matter. While the underlying electromagnetic wave theory simply scales with frequency and wavelength, we associate dramatically dissimilar phenomena with different regions of the spectrum, ranging all the way from audio signals that drive classical vibrations we can hear and feel to cosmic rays.
So what is it that distinguishes the THz spectral region: what kinds of science, technology, and applications have arisen, and what kinds of scientific and technological applications can we foresee? One THz corresponds to an energy of 0.004 eV, a temperature of 50 K, a wavelength of 0.3 mm, and, in common spectroscopic terms, 33.3 cm-1. If the commonly used bounds extending from perhaps 0.1 THz (near where a large proportion of current THz work is done) to 10 THz are adopted, the corresponding temperature scale ranges from 5 K to 500 K.
We will argue below that the relative size of hν and kT is important. Thus, these wide definitions of the THz regime include both the hν /kT >> 1 and hν /kT << 1 limits, with a correspondingly broad range of phenomena. Likewise, this definition of the THz includes wavelengths from 3 mm to 30µ. Thus, size considerations (whose scales are ordinarily set by the wavelength) lead to low order mode (i.e. microwave) devices in the longer wavelength portions of this region and to high order mode (i. e. laser) devices at the shorter wavelengths. For now, we will simply note here that jumping what is sometimes referred to as the “gap in the electromagnetic spectrum” is not equivalent to filling it.
B. What Phenomena Fall in this Energy Range? To a reasonable approximation the interactions of THz radiation with matter can be divided into three categories: interactions with low pressure gases, interactions with gases near atmospheric pressure, and interactions with liquids and solids. This separation is according to the Q of the resonances, with phenomena in the first category having a Q of ~106, in the second a Q of ~102, and in the last very low Q, or more often continuum interactions. Most of the successful applications of this spectral region fall into the first category and are dependent on the high Q for their success.
For an isolated system, its line-width Δν is related to its relaxation time Δτ by Δν~1/Δτ. For gasses this is a reasonable approximation and the concept of pressure broadening results from simple kinetic theory, with Δνpb typically ~10 MHz/Torr. At low pressure, Doppler broadening with Δν/ν~10-6 (1 MHz at 1 THz) sets a lower bound. However, in solids and liquids the resonant systems are not isolated or are often collective and the line-widths are much broader.
Because of the six orders of magnitude difference in the line-widths of THz phenomena, appropriate technology for the respective scientific studies varies widely, as does the basic physics of the phenomena involved. Thus, line-width provides a useful and convenient classification for both the sciences associated with the THz as well as the appropriate technology for their study.
C. Gases: Figure I -1 shows the strength of interactions as a function of frequency and molecular mass [7]. These absorptions result from the interaction of the rotation of the molecule with the radiation and exist only in gases. This figure shows that the strengths of the interactions increase as ν2 - ν3. As a result, THz interactions are 103 - 106 times more intense than interactions in the conventional Microwave (MW) region. Beyond a mass dependent peak in the THz, the interaction strength falls exponentially towards the infrared. This very sharp peak in the THz is one of the most important features of the spectral region and is closely related to many of the current and potential applications.
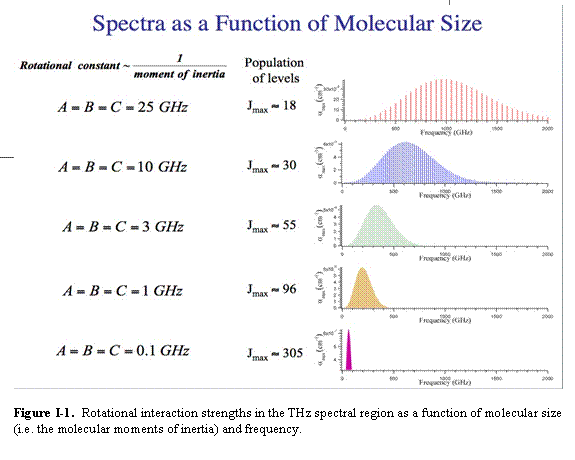 Figure I-2 shows the atmospheric propagation at sea level for a reasonably moist standard atmosphere [8]. This is both a spectroscopic problem of considerable interest and a factor of significant technological importance for THz systems. It is important to note that the vertical axis is a logarithmic plot of a logarithmic quantity and seemingly small differences are in fact very large. In this figure the rapid increase in absorption as we move out of the MW into the THz (due primarily to water and oxygen) can be seen. Figure I-2 also shows the atmospheric absorption as a function of altitude. This is a very important figure because it shows how rapidly atmospheric transmission changes as a function of altitude. For example, in windows around 500 GHz, at sea level the attenuation is ~ 100 db/km (virtually opaque), whereas at 16 km the attenuation is ~0.01 db/km (so small as to be difficult to detect).
Figure I-2 shows the atmospheric propagation at sea level for a reasonably moist standard atmosphere [8]. This is both a spectroscopic problem of considerable interest and a factor of significant technological importance for THz systems. It is important to note that the vertical axis is a logarithmic plot of a logarithmic quantity and seemingly small differences are in fact very large. In this figure the rapid increase in absorption as we move out of the MW into the THz (due primarily to water and oxygen) can be seen. Figure I-2 also shows the atmospheric absorption as a function of altitude. This is a very important figure because it shows how rapidly atmospheric transmission changes as a function of altitude. For example, in windows around 500 GHz, at sea level the attenuation is ~ 100 db/km (virtually opaque), whereas at 16 km the attenuation is ~0.01 db/km (so small as to be difficult to detect).
D. Liquids and Solids: All of the interactions discussed above are based on the interaction of molecular rotation with THz radiation. In liquids and solids there is no rotation and consequently no rotational spectra. However, for large molecules collective motions are possible which result in energy level spacings that correspond to THz frequencies. Solids and liquids are strongly interacting and as a result resonances are much broader, ordinarily leading to 'continua' spectra in the THz. However, evidence of specific features in biological substances has been reported. Although speculative, it would appear to us that such spectra are most likely at relatively high THz frequencies.
E. Applications and Impact of THz Spectroscopy: High resolution THz spectroscopy has had a major impact on many important fields of science and technology. The earliest studies in this region were of species such as H2O, O2, NO, CH3F, and OCS 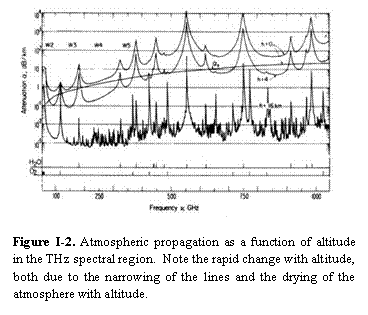 and served to both establish spectroscopic methodologies and to provide basic information about molecular structure and interactions [4, 9, 10]. A general understanding of the basic spectroscopic properties of these and the other small, fundamental species has been established [11-14]. Because these small, fundamental species have intrinsically interesting collisional properties, their dynamical properties have been studied as well. These studies have ranged from investigations of pressure broadening near room temperature (which are fundamental to the deconvolution of atmospheric remote sensing data) [15-18] to basic studies of the quantum nature of molecular collisions at low temperature [19-21].
and served to both establish spectroscopic methodologies and to provide basic information about molecular structure and interactions [4, 9, 10]. A general understanding of the basic spectroscopic properties of these and the other small, fundamental species has been established [11-14]. Because these small, fundamental species have intrinsically interesting collisional properties, their dynamical properties have been studied as well. These studies have ranged from investigations of pressure broadening near room temperature (which are fundamental to the deconvolution of atmospheric remote sensing data) [15-18] to basic studies of the quantum nature of molecular collisions at low temperature [19-21].
Because the strength of the interactions between electromagnetic radiation and molecular rotation peaks sharply in the THz, this spectral region has also been well suited for the study of reactive species such as free radicals [22-24] and ions [25-27] as well as weakly bound complexes [28, 29].Laboratory studies of molecular lasers and the collision induced rotational and vibrational processes which are central to their operation have also been important [30-32].
A variety of spectroscopically based remote sensing applications has grown out of this more basic work. Of these, two have become of major importance. The first is the study of the chemical processes in the upper atmosphere which are important in ozone formation and destruction [33-35]. Also, the vast majority of the over 100 molecular species which have been identified and studied in the interstellar medium have been observed by means of mm/submm ‘radio’ astronomy [36, 37]. We will discuss each of these in more detail below.
Because of these and other applications (e. g. the modeling of atmospheric propagation) the spectroscopic properties of virtually all of the important atmospheric and astronomical species have been collected into data bases. These data bases have become the standard for many applications and play an important role in the development of the spectral region. The Submillimeter, Millimeter, and Microwave Spectral Line Catalog (http://spec.jpl.nasa.gov/) has been maintained by the Jet Propulsion Laboratory for many years [13]. Likewise, The HITRAN Molecular Spectroscopic Database (http://www.hitran.com/) has been maintained by the Air Force [14]. While the latter began primarily as an infrared database, the growth in both infrared and submillimeter experimental technologies has been such that for many molecular species the best spectral data base results from a weighted fit of infrared and microwave data to a theoretical model.
Although most of the spectroscopic work in this spectral region historically has been referred to as millimeter and submillimeter spectroscopy, in this chapter we will for the most part use the term THz. An interesting study of the relationships among the communities that work in this spectral region can be done by using an Internet search engine to explore 'THz' and 'submillimeter' Boolean combined with 'spectroscopy'.
References
[1] W. Gordy, "Microwave Spectrocopy," Rev. Modern Phys., vol. 20, pp. 668-689, 1948.
[2] W. Gordy, "Microwave Spectroscopy above 60 KMc," N.Y. Academy of Science, vol. 55, pp. 744-788, 1952.
[3] C. A. Burrus Jr. and W. Gordy, "Submillimeter Wave Spectroscopy," Phys. Rev., vol. 93, pp. 897-898, 1954.
[4] W. C. King and W. Gordy, "One to Two Millimeter Wave Spectroscopy. I.," Phys. Rev., vol. 90, pp. 319-320, 1953.
[5] R. R. Unterberger and W. V. Smith, "A Microwave Secondary Frequency Standard," Rev. Sci. Inst., vol. 19, pp. 580-585, 1948.
[6] P. Helminger, F. C. De Lucia, and W. Gordy, "Extension of Microwave Absorption Spectroscopy to 0.37-mmWavelength,"Phys. Rev. Lett., vol. 25, pp.1397-1399,1970.
[7] S. Albert, D. T. Petkie, R. P. A. Bettens, S. P. Belov, and F. C. De Lucia, "FASSST: A new Gas-Phase Analytical Tool," Anal. Chem., vol. 70, pp. 719A-727A, 1998.
[8] H. J. Liebe, "Atmospheric Water Vapor: A Nemesis for Millimeter Wave Propagation," in Atmospheric Water Vapor, A. Deepak, T. D. Wilkerson, and L. H. Ruhnke, Eds. New York: Academic Press, 1980.
[9] C. A. Burrus Jr. and W. Gordy, "One-to-Two Millimeter Wave Spectroscopy. II. H2S," Phys. Rev., vol. 92, pp. 274-277, 1953.
[10] W. C. King and W. Gordy, "One-to-Two Millimeter Wave Spectroscopy. IV. Experimental Methods and Results for OCS,CH3F, and H2O," Phys. Rev., vol. 93, pp. 407- 412, 1954.
[11] W. Gordy, "Microwave Spectroscopy in the Region of 4-0.4 Millimeters," J. of Pure and Applied Chemistry, Vol. II,pp. 403-434, 1965.
[12] F. C. De Lucia, "Millimeter and Submillimeter-wave Spectroscopy," Molecular Spectroscopy, Modern Research, Vol. II, pp. 73-92, 1976.
[13] H. M. Pickett, R. L. Poynter, E. A. Cohen, M. L. Delitsky, J. C. Pearson, and H. S. P. Muller, "Submillimeter, Millimeter, and Microwave Spectral Line Catalog," J. Quant. Spectrosc. Rad. Transfer, vol. 60, pp. 883-890, 1998.
[14] L. S. Rothman, C. P. Rinsland, A. Goldman, S. T. Massie, D. P. Edwards, J.-M. Flaud, A. Perrin, C. Camy-Peyret,V. Dana, J.-Y. Mandin, J. Schroeder, A. Mccann, R. R. Gamache, R. B. Wattson, K. Yoshino, K. V. Chance, K. W. Jucks, L. R. Brown, V. Nemtchinov, and P. Varanasi, "The HITRAN Molecular Spectroscopic Database and HAWKS (HITRAN Atmospheric Workstation): 1996 Edition," J. Quant. Spectrosc. Radiat. Transfer, vol. 60, pp. 665- 710, 1998.
[15] P. Helminger and F. C. De Lucia, "Pressure Broadwning of Hydrogen Sulfide," J. Quant. Spectrosc. Radiat. Transfer, vol. 17, pp. 751-754, 1977.
[16] H. M. Pickett, "Determination of Collisional Linewidths and Shifts by a Convolution Method," Appl. Opt., vol. 19, pp. 745-2749, 1980.
[17] A. Bauer, M. Godon, M. Kheddar, J. H. Hartmann, J. Bonamy, and D. Robert, "Temperature and Perturber Dependences of the Water-Vapor 380 GHz-Line Broadening," J. Quant. Spectrosc. Radiat. Transfer, vol. 37, pp. 531, 1987.
[18] T. M. Goyette, F. C. De Lucia, J. M. Dutta, and C. R. Jones, "Variable Temperature Pressure Broadening of the 4 1 ,4 - 3 2,1 Transition of H2O by O2 and N2," J. Quant. Spectrosc. and Rad. Transfer, vol. 49, pp. 485-489, 1993.
[19] J. K. Messer and F. C. De Lucia, "Measurement of Pressure-Broadening Parameters for the CO-He System at 4 K," Phys. Rev. Lett., vol. 53, pp. 2555-2558, 1984.
[20] J. C. Pearson, L. C. Oesterling, E. Herbst, and F. C. De Lucia, "Pressure Broadening of Gas Phase Molecular Ions at Very Low Temperature," Phys. Rev. Lett., vol. 75, pp. 2940-2943, 1995.
[21] C. D. Ball and F. C. De Lucia, "Direct Measurement of Rotationally Inelastic Cross Sections at Astrophysical and Quantum Collisional Temperatures," Phys. Rev. Lett.,vol. 81, pp. 305-308, 1998.
[22] M. Winnewisser, K. V. L. N. Sastry, R. L. Cook, and W. Gordy, "Millimeter Wave Spectroscopy of Unstable Molecular Species II. Sulfur Monoxide," J. Chem. Phys.,vol. 41, pp. 1687-1691, 1964.
[23] K. M. Evenson, R. J. Saykally, D. A. Jennings, R. F. Curl, and J. M. Brown, Far Infrared Laser Magnetic Resonance, vol. V. New York: Academic, 1980.
[24] A. Charo and F. C. De Lucia, "The Millimeter and Submillimeter Spectrum of HO2: The Effects of the Unpaired Electronic Spin in a Light Asymmetric Rotor," J. Mol. Spectrosc., vol. 94, pp. 426-436, 1982.
[25] R. C. Woods, T. A. Dixon, R. J. Saykally, and P. G. Szanto, "Laboratory Microwave Spectrum of HCO+," Phys. Rev. Lett., vol. 35, pp. 1269-1272, 1975.
[26] F. C. van den Heuvel and A. Dynamus, "Observation of Far-Infrared Transitions of HCO+, CO+, and NH2+," Chem. Phys. Lett., vol. 92, pp. 219-222, 1982.
[27] F. C. De Lucia, E. Herbst, G. M. Plummer, and G. A. Blake, "The Production of Large Concentrations of Molecular Ions in the Lengthened Negative Glow Region of a Discharge," J. Chem. Phys., vol. 78, pp. 2312-2316, 1983.
[28] K. L. Busarow, G. A. Blake, K. B. Laughlin, R. C. Cohen, Y. T. Lee, and R. J. Saykally, "Tunable Far-Infrared Laser Spectroscopy in a Planar Supersonic Jet: The S Bending Vibrations of Ar-HCl," Chem. Phys. Lett., vol. 141, pp. 289-291, 1987.
[29] M. D. Marshall, A. Charo, H. O. Leung, and W. Klemperer, "Characterization of the lowest-lying P bending state of Ar-HCl by far infrared laser-Stark spectroscopy and molecular beam electric resonance," J. Chem. Phys., vol. 83, pp. 4924-4933, 1985.
[30] F. C. De Lucia, "The Study of Laser Processes by Millimeter and Submillimeter Microwave Spectroscopy," Appl. Phys. Lett., vol. 31, pp. 606-608, 1977.
[31] D. D. Skatrud and F. C. De Lucia, "Dynamics of the HCN Discharge Laser," Appl. Phys. Lett., vol. 46, pp. 631-633, 1985.
[32] H. O. Everitt, D. D. Skatrud, and F. C. De Lucia, "Dynamics and Tunability of a Small Optically Pumped CW Far-Infrared Laser," Appl. Phys. Lett., vol. 49, pp. 995-997, 1986.
[33] K. V. Chance, D. G. Johnson, W. A. Traub, and K. W. Jucks, "Measurement of the Stratospheric Hydrogen Peroxide Concentration Profile using Far Infrared Thermal Emission Spectroscopy," Geophys. Res. Letts., vol. 18, pp. 1003, 1991.
[34] J. W. Waters, Atmospheric Remote Sensing by Microwave Radiometry. Wiley, New York, 1993.
[35] B. Carli and J. H. Park, "Simultaneous Measurement of Minor Stratospheric Constituents with Emission Far-Infrared Spectroscopy," J. Geophys. Res., vol. 93,pp. 3851, 1988.
[36] E. Herbst, "Chemistry in the Interstellar Medium," Ann. Rev. Phys. Chem., vol. 46, pp. 27, 1995.
[37] T. G. Phillips, in The Physics and Chemistry of Interstellar Molecular Clouds, G. Winnewisser and G. C. Pelz, Eds. New York: Springer, 1995, pp. 344.
Back to home page
 Figure I-2 shows the atmospheric propagation at sea level for a reasonably moist standard atmosphere [8]. This is both a spectroscopic problem of considerable interest and a factor of significant technological importance for THz systems. It is important to note that the vertical axis is a logarithmic plot of a logarithmic quantity and seemingly small differences are in fact very large. In this figure the rapid increase in absorption as we move out of the MW into the THz (due primarily to water and oxygen) can be seen. Figure I-2 also shows the atmospheric absorption as a function of altitude. This is a very important figure because it shows how rapidly atmospheric transmission changes as a function of altitude. For example, in windows around 500 GHz, at sea level the attenuation is ~ 100 db/km (virtually opaque), whereas at 16 km the attenuation is ~0.01 db/km (so small as to be difficult to detect).
Figure I-2 shows the atmospheric propagation at sea level for a reasonably moist standard atmosphere [8]. This is both a spectroscopic problem of considerable interest and a factor of significant technological importance for THz systems. It is important to note that the vertical axis is a logarithmic plot of a logarithmic quantity and seemingly small differences are in fact very large. In this figure the rapid increase in absorption as we move out of the MW into the THz (due primarily to water and oxygen) can be seen. Figure I-2 also shows the atmospheric absorption as a function of altitude. This is a very important figure because it shows how rapidly atmospheric transmission changes as a function of altitude. For example, in windows around 500 GHz, at sea level the attenuation is ~ 100 db/km (virtually opaque), whereas at 16 km the attenuation is ~0.01 db/km (so small as to be difficult to detect). and served to both establish spectroscopic methodologies and to provide basic information about molecular structure and interactions [
and served to both establish spectroscopic methodologies and to provide basic information about molecular structure and interactions [