Research Overview
 We aim to understand what happens when cells physically interact with their environment-specifically, how their eating habits (endocytosis) are influenced and how this impacts various cellular functions.
We're also interested in uncovering the mechanical properties of cells and predicting their next moves-whether they will divide, migrate, or even self-destruct-by analyzing their eating patterns.
We aim to understand what happens when cells physically interact with their environment-specifically, how their eating habits (endocytosis) are influenced and how this impacts various cellular functions.
We're also interested in uncovering the mechanical properties of cells and predicting their next moves-whether they will divide, migrate, or even self-destruct-by analyzing their eating patterns.
 o Clathrin-mediated endocytosis (CME) is the primary mechanism by which membrane lipids and proteins are internalized from the cell surface. Over the years, various biophysical and biochemical methods have been used to study the structural and dynamic properties of endocytic clathrin coats. However, fundamental aspects of CME remain debated due to the lack of experimental approaches that directly link the ultrastructural and dynamic properties of clathrin coats.
To address this, we develop innovative experimental and analytical techniques to study the structure and dynamics of clathrin-coated structures both in vitro (in cultured cells) and in vivo (in tissues of multicellular organisms).
o Clathrin-mediated endocytosis (CME) is the primary mechanism by which membrane lipids and proteins are internalized from the cell surface. Over the years, various biophysical and biochemical methods have been used to study the structural and dynamic properties of endocytic clathrin coats. However, fundamental aspects of CME remain debated due to the lack of experimental approaches that directly link the ultrastructural and dynamic properties of clathrin coats.
To address this, we develop innovative experimental and analytical techniques to study the structure and dynamics of clathrin-coated structures both in vitro (in cultured cells) and in vivo (in tissues of multicellular organisms).
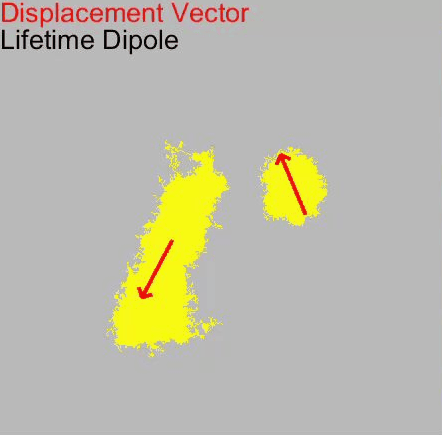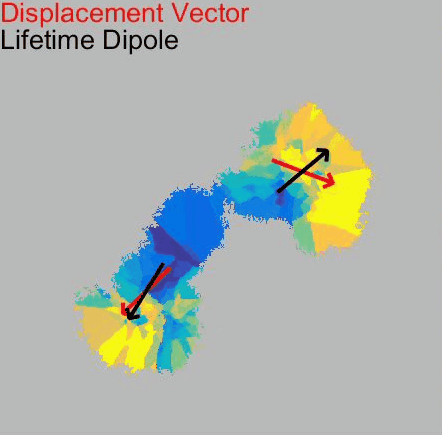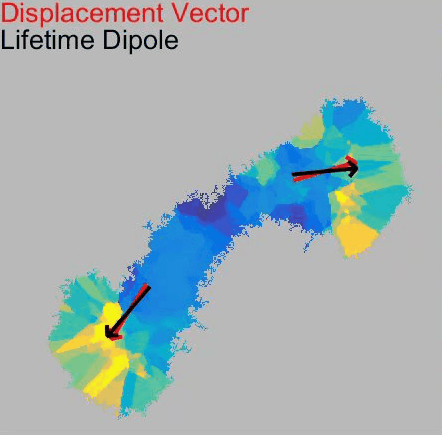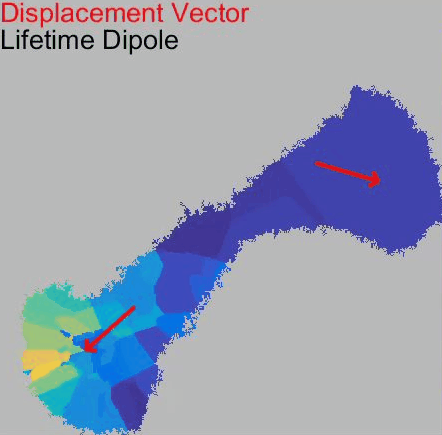
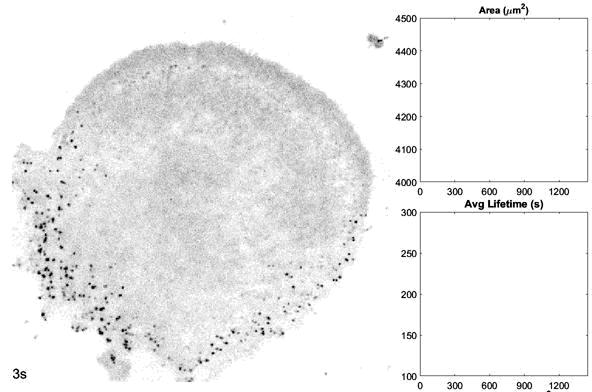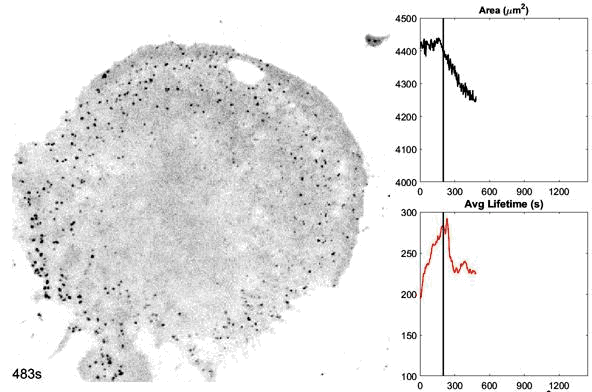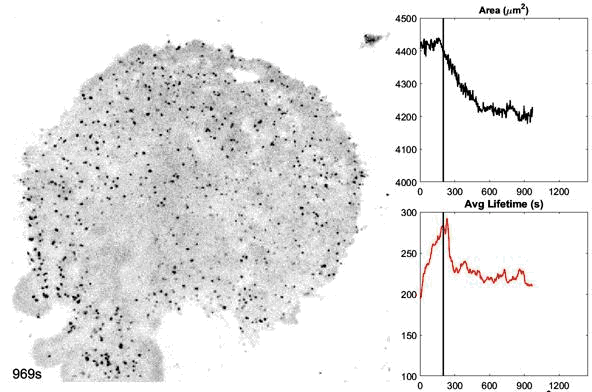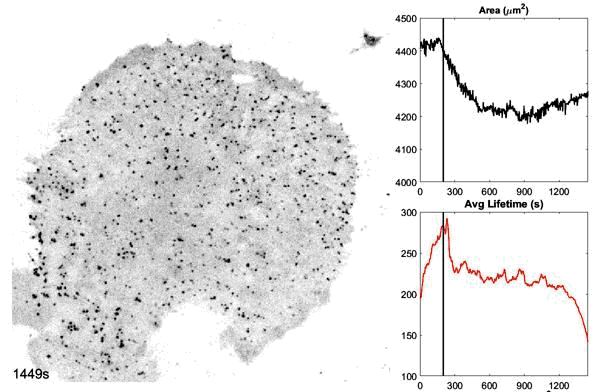 o The dynamics and structures of endocytic clathrin coats vary remarkably-not just across different cell types, but even within the same culture or on different regions of a single cell. We have shown that this spatiotemporal heterogeneity in CME dynamics becomes particularly pronounced during cell division, migration, and spreading. Changes in CME rates contribute to increased proliferation, migration, and metastasis of cancer cells. However, the mechanisms driving this heterogeneity-which regulates endocytosis during key cellular and developmental processes-remain unclear.
To investigate this, we focus on the clathrin adaptors AP2, FCHo, and CALM to test our hypotheses. Our research aims to:
i) determine the recruitment dynamics and stoichiometric ratios of adaptors to clathrin coats under different membrane tension levels,
ii) uncover the distinct roles of curvature-generating clathrin adaptors in maintaining CME under varying tension conditions,
iii) understand how spatiotemporal CME heterogeneity enables cell migration, spreading, and division.
o The dynamics and structures of endocytic clathrin coats vary remarkably-not just across different cell types, but even within the same culture or on different regions of a single cell. We have shown that this spatiotemporal heterogeneity in CME dynamics becomes particularly pronounced during cell division, migration, and spreading. Changes in CME rates contribute to increased proliferation, migration, and metastasis of cancer cells. However, the mechanisms driving this heterogeneity-which regulates endocytosis during key cellular and developmental processes-remain unclear.
To investigate this, we focus on the clathrin adaptors AP2, FCHo, and CALM to test our hypotheses. Our research aims to:
i) determine the recruitment dynamics and stoichiometric ratios of adaptors to clathrin coats under different membrane tension levels,
ii) uncover the distinct roles of curvature-generating clathrin adaptors in maintaining CME under varying tension conditions,
iii) understand how spatiotemporal CME heterogeneity enables cell migration, spreading, and division.
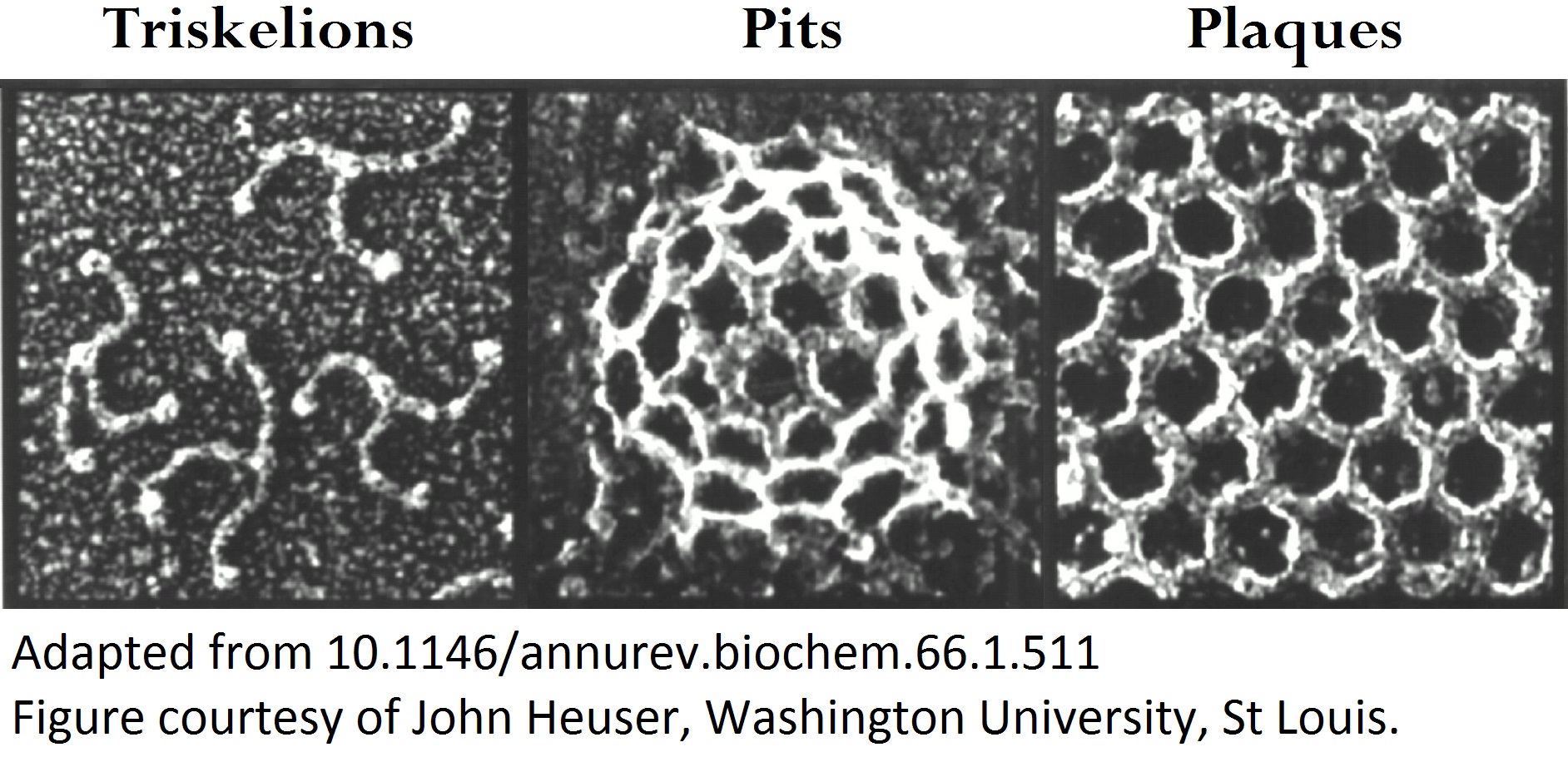
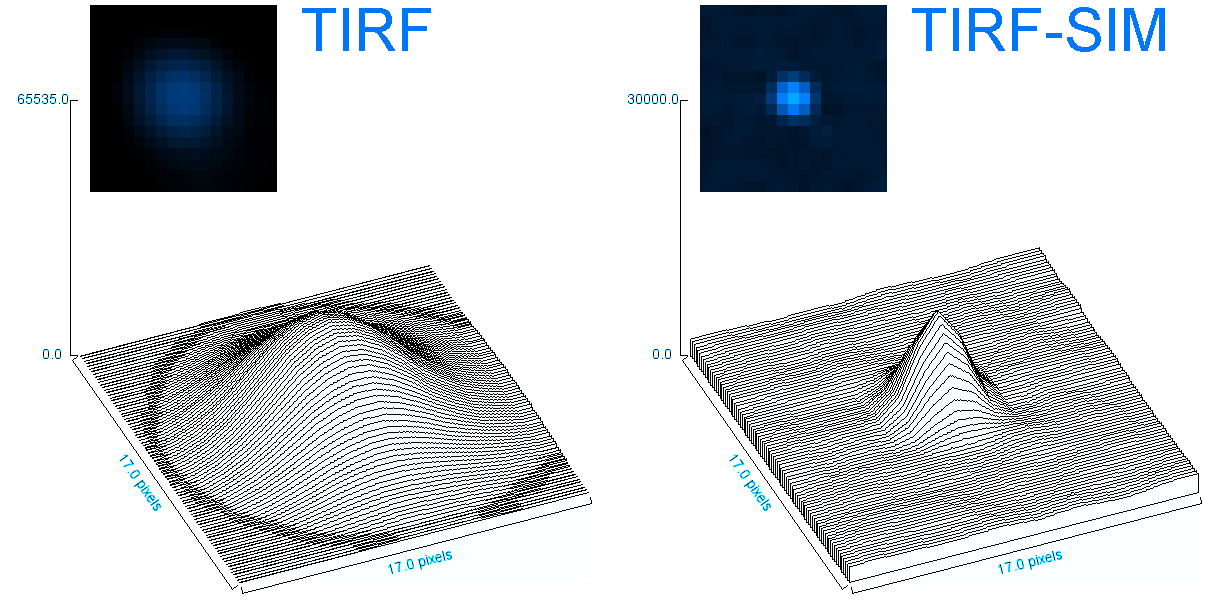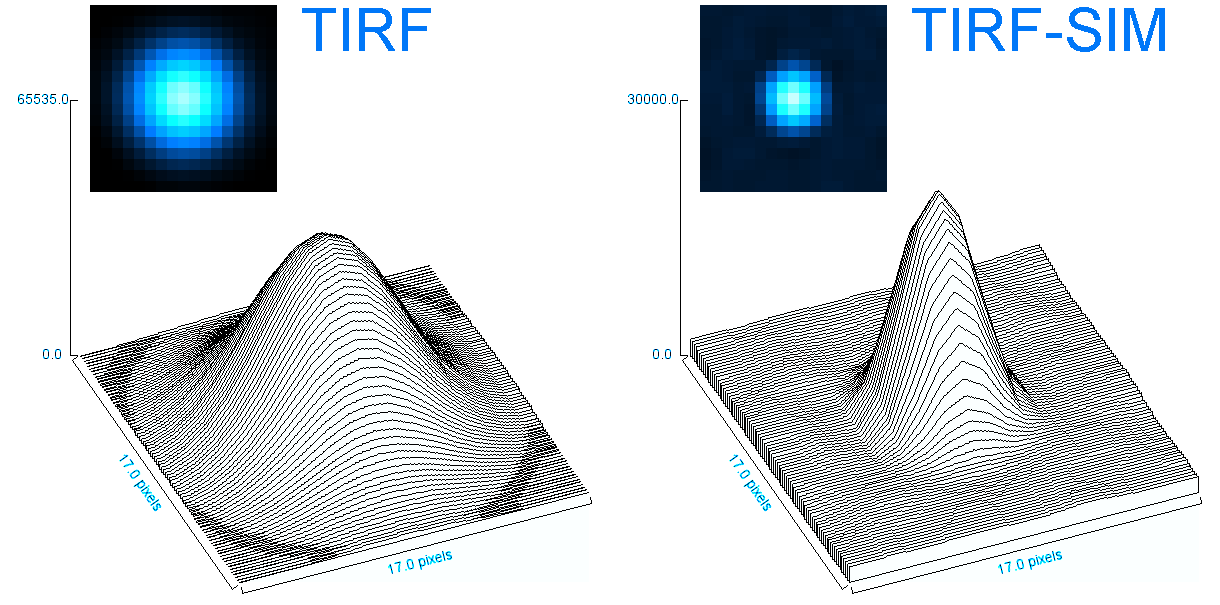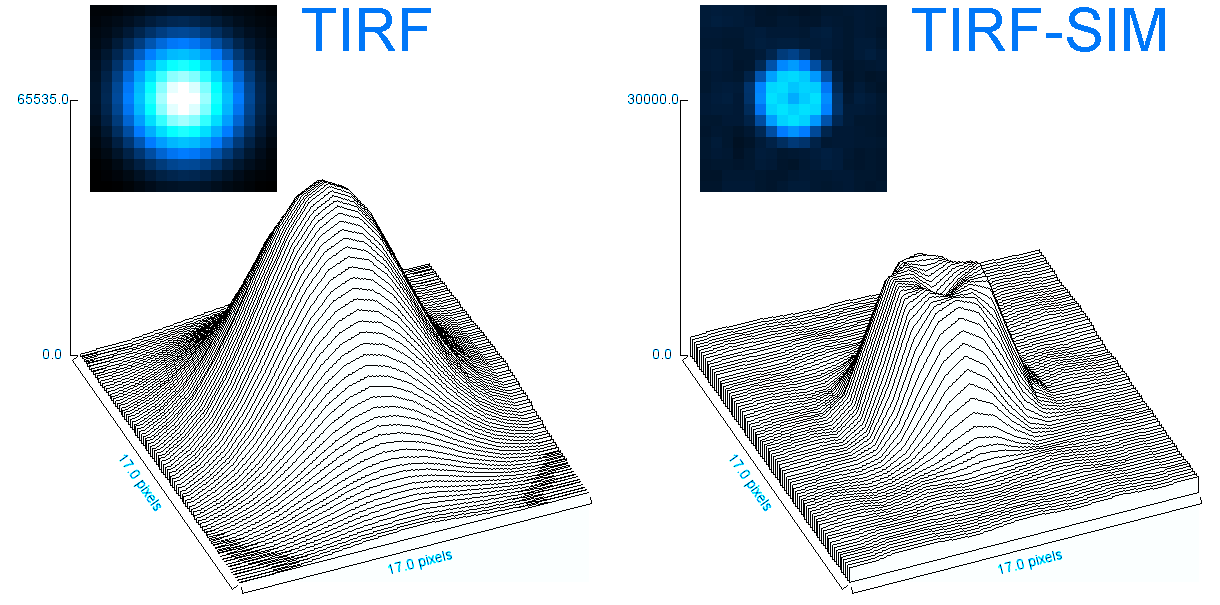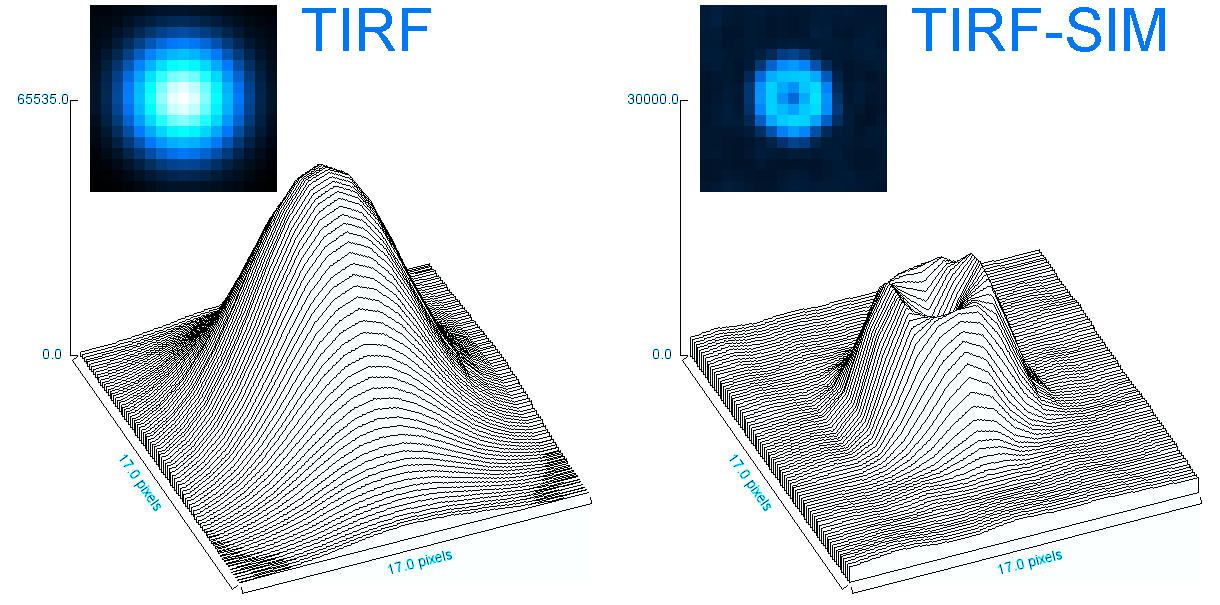 o Clathrin triskelions can assemble into membrane-bound coats-forming polyhedral cages and lattices-in an almost limitless number of geometries. Regardless of their shape or size, endocytic vesicle formation requires the generation of curvature throughout the lifespan of clathrin coats. However, when and how this curvature develops remains an open question.
Electron microscopy provides high-resolution snapshots of clathrin-coated structures at different curvature stages. However, because these images lack a temporal dimension, they cannot directly reveal the mechanism of clathrin-driven curvature generation. To address this, we use various super-resolution fluorescence techniques to study curvature formation in different classes of clathrin-coated structures in live cells and tissues.
o Clathrin triskelions can assemble into membrane-bound coats-forming polyhedral cages and lattices-in an almost limitless number of geometries. Regardless of their shape or size, endocytic vesicle formation requires the generation of curvature throughout the lifespan of clathrin coats. However, when and how this curvature develops remains an open question.
Electron microscopy provides high-resolution snapshots of clathrin-coated structures at different curvature stages. However, because these images lack a temporal dimension, they cannot directly reveal the mechanism of clathrin-driven curvature generation. To address this, we use various super-resolution fluorescence techniques to study curvature formation in different classes of clathrin-coated structures in live cells and tissues.







