the amorphous silica/water interface
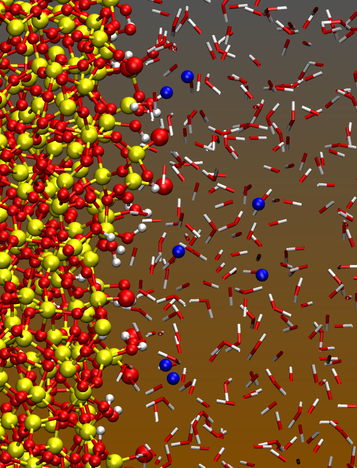 Interfaces between amorphous silica and water are
ubiquitous in chemical, biochemical and environmental settings. The
strong interactions between silica, water, and adsorbates, such as
biomolecules, make the amorphous silica surface an important and
challenging system to model in several arenas. On a most mundane
level, laboratory glassware is about three-quarters amorphous
SiO2. Silica is also one of the most important materials
for chromatography. Silica-DNA interactions are the basis of a
standard purification scheme for nucleic acids. Novel micro- and
nano-scale devices are often fabricated from amorphous silica, or from
silicon, which acquires an oxide coating in contact with water. There
has been recent interest in using silica nanochannels to stretch and
sequence DNA.
Interfaces between amorphous silica and water are
ubiquitous in chemical, biochemical and environmental settings. The
strong interactions between silica, water, and adsorbates, such as
biomolecules, make the amorphous silica surface an important and
challenging system to model in several arenas. On a most mundane
level, laboratory glassware is about three-quarters amorphous
SiO2. Silica is also one of the most important materials
for chromatography. Silica-DNA interactions are the basis of a
standard purification scheme for nucleic acids. Novel micro- and
nano-scale devices are often fabricated from amorphous silica, or from
silicon, which acquires an oxide coating in contact with water. There
has been recent interest in using silica nanochannels to stretch and
sequence DNA.
At all but the lowest pH values, a silica surface in contact with water is negatively charged because some silanol groups dissociate (-Si-O-H → -Si-O-). Most of the compensating charge of the aqueous electrolyte is typically located within a nanometer from the surface. Description of this region is crucial for understanding and predicting behavior in modern nano-scale devices. It is also helps resolve classic problems in physical chemistry dating back to the early 20th century.
There is a need for interaction potential models that can describe this region with some realism, and yet will be tractable for simulations that cover large spatial and time scales. We have developed a successful interaction model that reproduces known experimental data like the enthalpy for immersing silica in water, and also compares will to ab initio benchmarks. Please see the description of our work on electrokinetic phenomena for our most important applications.
This research is supported by the National Science Foundation.
some relevant publications
- Ali A. Hassanali and Sherwin J. Singer, ``Static and dynamic properties of the water/amorphous silica interface: a model for the undissociated surface'', J.Computer-Aided Materials Design, 14(1):53 (2007).
- Ali Hassanali and Sherwin J. Singer, ``A model for the water/amorphous silica interface: the undissociated surface'', J. Phys. Chem. B111(38):11181 (2007).
- Ali A.Hassanali, Hui Zhang, Chris Knight, Yun Kyung Shin and Sherwin J. Singer, ``The dissociated amorphous silica surface: Model development and evaluation'', J. Comput. Theoretical. Chem. 6 (11):3456 (2010).
- Hui Zhang, Ali A.Hassanali, Yun Kyung Shin, Chris Knight and Sherwin J. Singer, ``The water-amorphous silica interface: Analysis of the Stern layer and surface conduction'', J. Chem. Phys. 134(2):024705 (2011).